The kit we bought for our farm was obtained from maggidans.com http://www.maggidans.com/kte.htm
The Deluxe microscope has a mechanical stage so that you do not have to move the slide in order to examine it. It also has a rechargeable light. This kit also comes with supplies to run several other advanced tests, and a handy guide to identifying different types of organisms and eggs. I highly recommend this kit, which is priced at $184.95.
In this tutorial, we will discuss the process for preparing and examining a simple fecal sample using the Deluxe kit from Maggidans. I will also be discussing other ways to prepare samples for examinations using other supplies. Let's get started!
First, let's discuss collection of the sample.
The most efficient way to collect a "clean" sample is to go in and get it. This method ensures that the sample is not contaminated by outside factors. I use a surgical glove and some lube or soapy water for lubrication. Insert a gloved and lubed finger into the anus of the goat and pull out a couple of berries. Don't worry, this is not an exploratory mission. The berries will be right there and you do not have to go look for them. You only need two or three berries for a good sample. I put the sample directly into a plastic bag that has been labeled with the goat's name.
Tip: When collecting a sample, I always have the bag ready for collection first before getting the goat. Once you put the goat on the stanchion, wait a few minutes before "going in." In my experience, the goat will usually eliminate before you have to go in and retrieve. It's especially effective if you sweep (and mop!) the area under the stanchion first, because we all know that goats just LOVE to dirty a clean floor. HA! If this happens, simply open the baggie and let some poo drop into it.
Next, let's discuss supplies.
1. The first thing you need to do is to break up the feces in the bag. Use your fingers to tear up the berries through the bag, and break them into smaller pieces.This makes the liquification process easier.
2. Insert the green insert of the fecalyzer into the plastic bag and collect some feces in the small end of the fecalyzer insert. Do not pack the feces into it as that is counterproductive. The goat is to break up and liquify the feces, so keep it loose within the collection chamber.
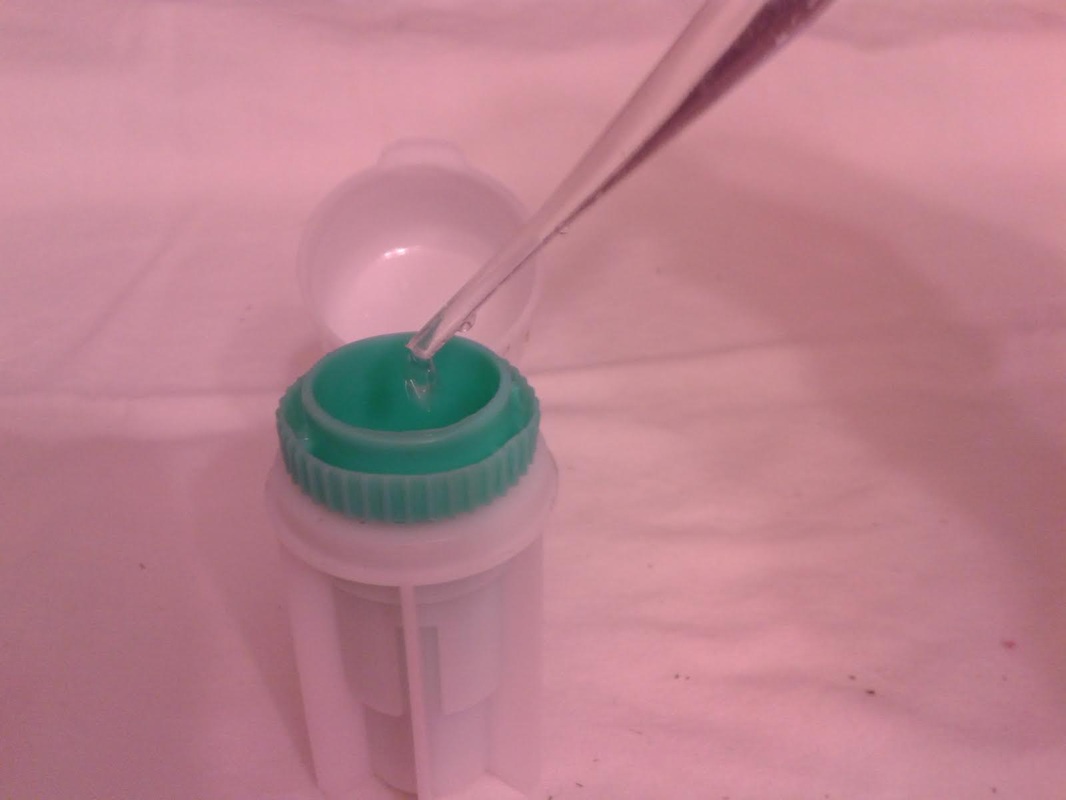
3. Place the green insert back into the fecalyzer container with the sample to the bottom of the container. Add two droppers full of the fecal floatation solution to the container to start to dissolve and stir the sample. The fecalyzer has an arrow on the side of the container. This is the initial fill level of the container.
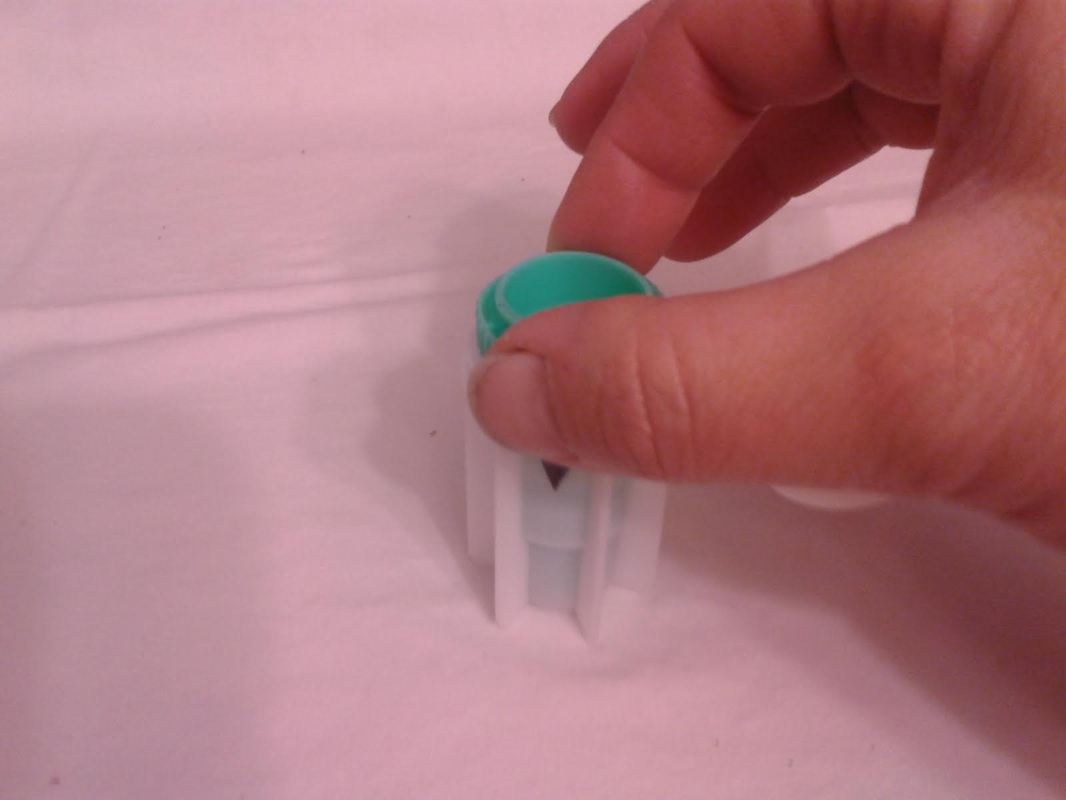
4. Seat the green insert back into the fecalyzer. Twist the green insert back and forth in the fecalyzer to stir and break up the sample. The insert has a stop in the end, so it will only turn 180 degrees in each direction. Let the sample sit for five minutes after twisting and then twist again to ensure it is mixed well. If you carefully lift the basket, your sample should look like muddy water. You may also see some solid plant material floating on top. The fecalyzer has a strainer basket on it that keeps some materials from rising to the top , so if you are not using the fecalyzer, you can use some cheesecloth or a tea strainer to strain the debris out of the sample before examination. Some debris in the final sample is to be expected.
With your eye dropper, add enough saline solution to create a meniscus (a bulge of water that extends up over the edge of the container). Be careful not to overflow the sample, but make sure you have enough so that it creates a convex surface for the slide cover to rest on.
6. After 15-20 minutes, you are now ready to transfer your sample to the slide. Lift straight up as not to disturb any eggs which have collected, and place it wet side down onto a slide.
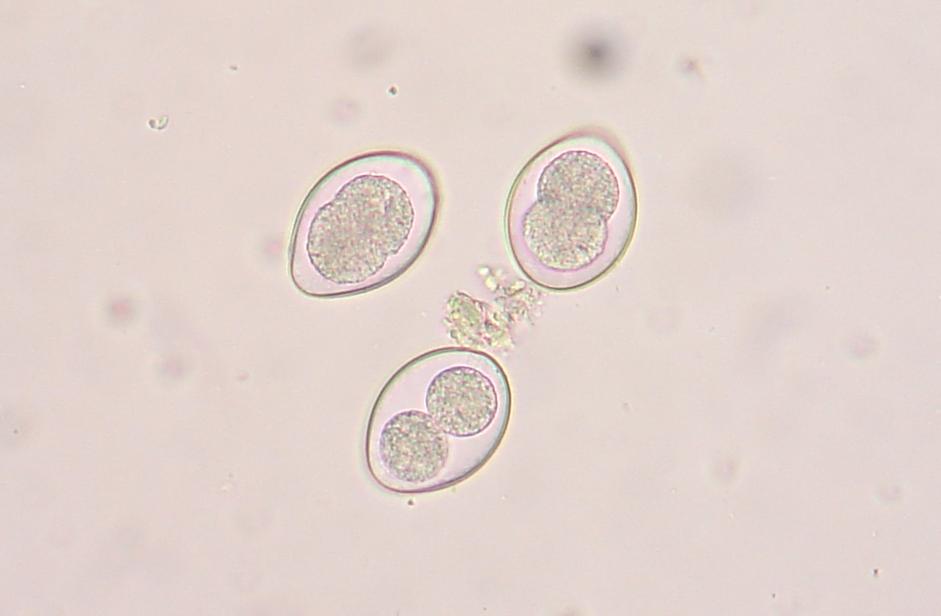
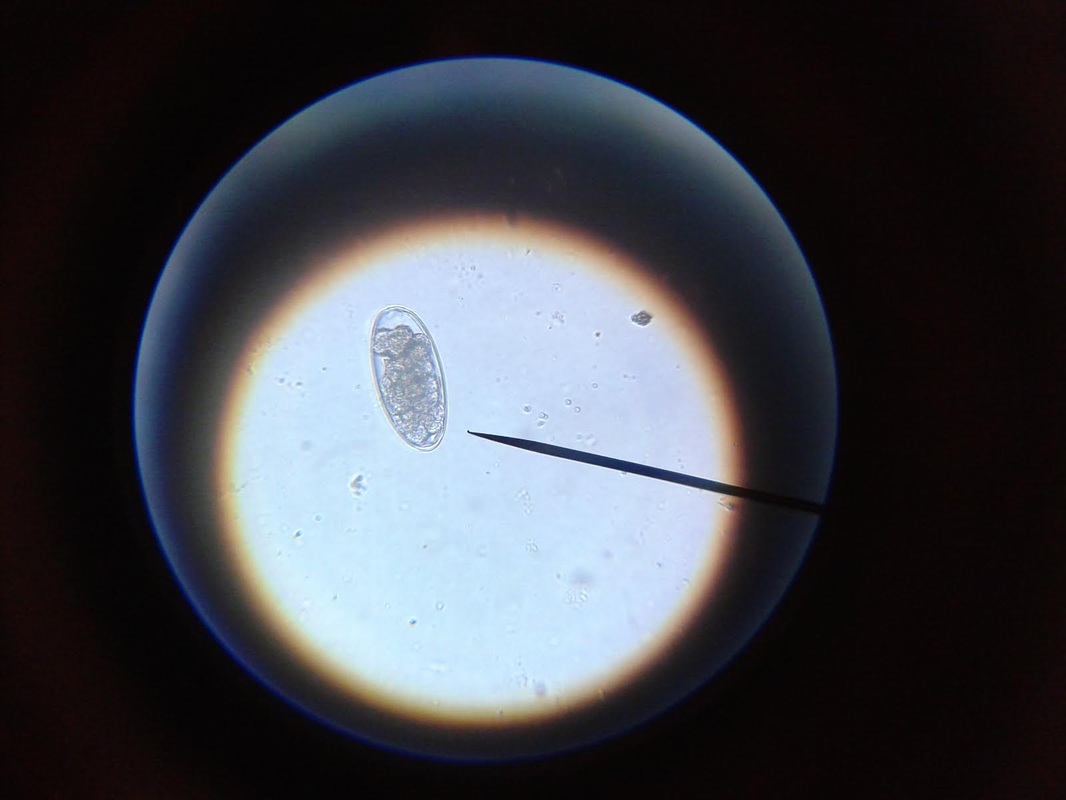
You will see many interesting things in the sample. There will be alot of plant matter (hay), air bubbles, curly looking fibers (also hay), and perhaps some salt crystals (which have edges and are angular). If your sample starts to dry during exam, you might see some very long thin looking salt crystals, that grow like frost. If it inhibits the view too much, start another sample. If you think you might see a parasite egg, but you are not sure, switch to a higher magnification (40X) and let in more light on the dial and try to focus on the nucleus of the egg. There are many different kinds of parasite eggs that you might find, but the most common and greatest threat will be coccidia and Haemonchus contortus (Barberpole). Coccidia is a protozoa that can affect any animal, and the first symptom is usually diarrhea (sometimes bloody.) Coccidia must be treated promptly or it can cause death.
I hope you enjoyed this tutorial, and I hope that you learned something from it. I will be adding the tutorial on FAMACHA testing very soon. Stay tuned!





















 RSS Feed
RSS Feed